Login
Displaying, validating, reviewing and scoring ECG files
The FDAEcg Suite is an advanced Viewer for resting ECGs in FDA HL7 XML format.
This AMPS software provides a way to display, review, score and validate ECG files in the format supported by the US FDA (aECG FDA HL7 XML, v. 1) and in ISHNE formatted ECG files.
With FDAEcg Suite users can customize the ECG display for Rhythm strip (raw data), and Representative Beats (medians) waveforms. Additionally, the Suite includes Validation and Scoring modules.
The Validation module confirms both the structure and content of the HL7 XML files meet the requirements of HL7 Schema and the HL7 Implementation Guide endorsed by the FDA. Reference: see HL7 aECG Implementation Guide and Beyond the Schema documents available on the Technical documents page on our website.
Thanks to the strategic alliance signed with Mortara Instruments Inc. in August 2006, FDAEcg Suite and FDAEcgWarehouse produce a consistent set of syntactical warnings and error messages during the validation phase, producing a consistent interpretation of the HL7 XML standard requirements.
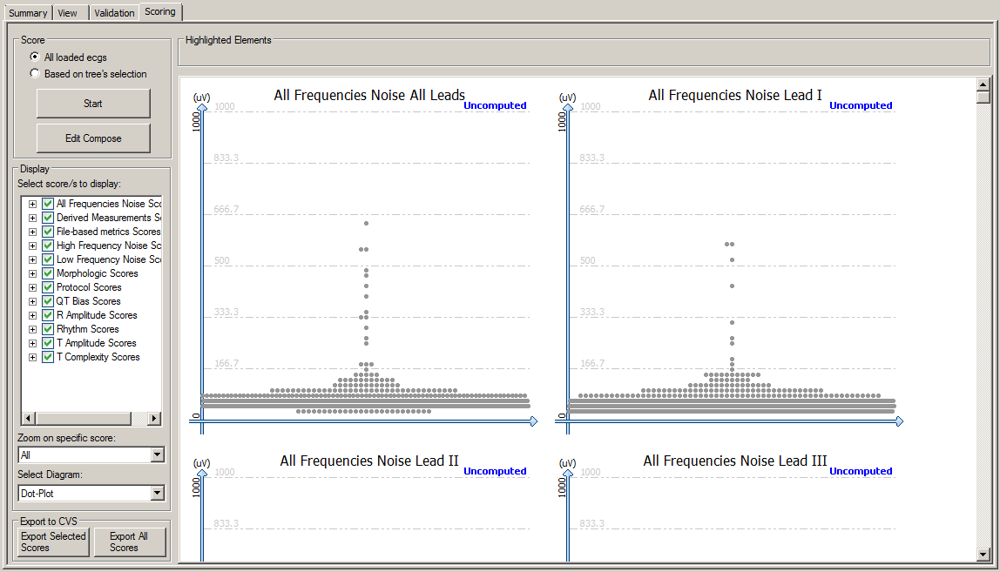
The Scoring module measures the quality of the loaded ECGs, using several predefined metrics, such as:
- Heart rate
- QRS complexes heterogeneity
- Protocol agreement percentage
- Following annotation based metrics: QT, QTcB, QTcF
- Lead specific (I, II, III, aVR, aVL, aVF, V1…V6) and all leads LF noise
- Lead specific and all leads HF noise & All frequencies noise
- Lead specific HF noise around T-wave offset
- Lead specific T-wave amplitude
History: Since January 20th, 2004, the Annotated ECG (aECG) XML Waveform Standard becameformally adopted by the Health Level 7 (HL7) standards organization. HL7's adoption of the aECG XML Waveform Standard enabled submission of digital aECGs to establish the cardiac safety of new drugs. The final aECG Waveform Standard was authored through an ad hoc group consisting of representatives from sponsor organizations, ECG core laboratories, academic institutions and medical device companies.Dr. Fabio Badilini, AMPS founder, was part of the panel and received an Award from the FDA for his contribution.
The FDAEcg Suite is fully modular, the Viewer, Validation and Score modules can be combined independently: any combination of the three modules are possible:
|
|
Viewer Light |
FDAEcg Suite |
Notes |
|
Against Schema Validation |
ü |
ü |
This validation checks if all used elements conform to the XML syntax rules and to the HL7 Schema. |
|
IG Level Validation |
û |
ü |
This validation checks if all used elements conform to the XML syntax rules and to the HL7 Schema. The IG (and this validator) presents information about the aECG standard independently of any particular use. |
|
FDA Warehouse Validation |
û |
ü |
This level is specifically aimed to address all issues related with the submission of the aECG files into the FDA warehouse, but that are NOT specified at the level of the schema (or even at the level of the IG). |
|
Folder-based validation |
û |
ü |
Validation can be computed on a single file or on an entire folder in a single step. |
|
Folder-based file management |
û |
ü |
The user can open the whole content browsing from an ECG to another without the need of additional open sessions. |
|
The application gives the user the possibility to browse between ECGs and enrolled subjects of the same study, with identical trial code. |
|||
|
Enhanced display options |
ü |
ü |
Advanced and totally customizable ECG display. Possibility of changing lead organization, grid appearance and dimensions, zoom… |
|
Toggle on/off annotations |
ü |
ü |
It is possible to turn on and off the display of annotations by type. |
|
ECG Scoring |
û
|
ü
|
ECGs can be scored using pre-defined or imported plug-ins. Once scored the ECGs can be visualized and sorted based on the different scores, and reviewed. The Suite comes with an open user interface whereby it will be possible to add (plug-in) criteria for new metrics. |
FDAEcg Suite Plug-in
Click here to view a slide presentation of the FDAEcg Suite.
Ask for a Sample
Please fill out this form to request the Demo version of the FDAEcg Suite.
Please note that all the Demo versions available via the AMPS web site are fully functional only with the example demo data files included in the setup package. You will not be able to use the Demo version with your own files. A User Manual is included in the setup package, automatically copied in the installation folder.
In order to prevent abuse of this facility we require a valid e-mail address to be provided in order to allow the download of the demo version.
The password needed to open the demo installation package will be sent to the e-mail address you provide in the form.
We are painfully aware of the spam problems: AMPS will not sell or distribute your email address to third parties. The collection of this information by AMPS is intended for internal record keeping. All collected information will be kept strictly confidential.
An e-mail will be sent to you including a private link to download the demo you requested. If you have any specific question about this product please add them in the "Note" field.
FDAEcg Suite Demo
Request demo